Abstract
Background
Hemophilia is an X-linked bleeding disorder caused by mutations in blood clotting factor VIII [FVIII, hemophilia A (HA)] or factor IX [FIX, hemophilia B (HB)]. Treatment includes plasma-derived or recombinant (standard half-life [SHL] or extended half-life [EHL]) factor replacement, non-factor therapy and gene therapy (in development). The availability of newer and improved concentrates has resulted in switching between different plasma-derived or recombinant concentrates. Due to sparse information on products switching in hemophilia patients, this study provides more data to fill this gap based on current routine clinical practices using US EHR database.
Objectives
To describe switching patterns and associated bleeding outcomes in hemophilia patients in the US.
Methods
This is a retrospective cohort study using data from Optum EHR database from January 1, 2007 to September 30, 2019 in the US. The study population comprises all patients with at least 1 inpatient diagnosis or 2 outpatient evaluation and management (EM) diagnoses with International Classification of Diseases (ICD), 9th Revision, Clinical Modification (CM) (ICD-9-CM) code 286.0 and 286.1 or ICD-10-CM code D66 and D67 for HA and HB, respectively, and with a record for one of the specific drugs/procedures for hemophilia. The index date is the first date of prescription of prophylactic treatments for HA or HB in hemophilia patients during study period. "Switch" is defined as evidence of conversion to at least 3 consecutive new drug usage. Descriptive analyses include frequency and incidence of switching between different factor concentrates, and bleeding rate.
Results
The study included 2,388 HA and 739 HB patients, with mean age of 30.6 (range: 0 - 87) and 33.8 (range: 0 - 86) years, with 90% and 87% male, 12% and 10% of patients with hepatitis C history, 6% and 3% with human immunodeficiency virus (HIV) history, and 1% and 5% with bypassing agent at index date in HA and HB patients, respectively.
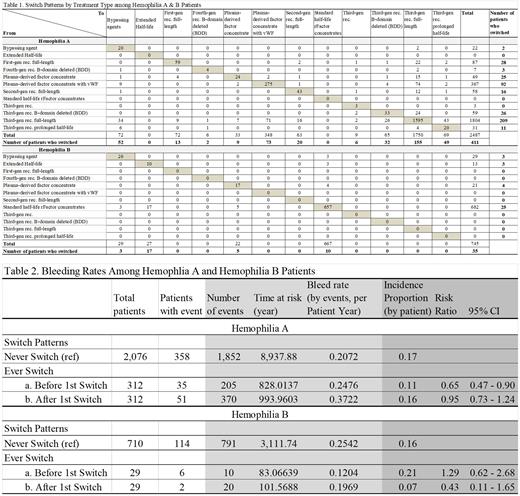
Three hundred and twelve (312) HA patients had 411 switches, with the majority from third-generation recombinant full-length products (TGRFL) (209 switches, 51%) and plasma-derived factor concentrate with vWF (PDFCvWF) (92 switches, 22%). There were 155 switches (38%) to TGRFL, 73 (18%) to PDFCvWF (the majority switched from third-gen rec. full-length), 52 (13%) to bypassing agents, 49 (12%) to EHL rFVIII products and 32 (8%) to third-gen B-domain deleted (BDD) SHL products, and 50 (11%) to others.
Twenty nine (29) HB patients had 35 switches. The majority (25 switches) were recorded from SHL rFIX concentrates (71%). Among the 35 switches, 17 (49%) were switched to EHL rFIX products and 10 (29%) to SHL rFIX products, and 8 (22%) to others.
Among 2,076 HA and 710 HB patients who never switched, 1,852 and 791 bleeding events were recorded in 358 (17%) HA and 114 (16%) HB patients, respectively. Among 312 HA and 29 HB patients who did switch, 205 and 10 bleeding events were recorded in 35 (11%) and 6 (21%) patients before switch (bleeding rates by events per patient year of 0.2476 in HA and 0.1204 in HB), while 370 and 20 events recorded in 51 (16%) and 2 (7%) patients after switch (bleeding rates 0.3722 in HA and 0.1969 in HB).
Compared to patients who never switched, the risk of bleeding was lower for HA patients before switching (Risk Ratio (RR)=0.65, 95% CI: 0.47-0.90) and similar after switching (RR=0.95, 95% CI: 0.73-1.24); for HB the risk was similar before switching (RR=1.29, 95% CI: 0.62-2.68) and numerically lower after switching (RR=0.43, 95% CI: 0.11-1.65).
Conclusions
Among HA patients, the majority of switches occurred from and to TGRFL and PDFCvWF products. Among HB patients, about half switches occurred from SHL rFIX to EHL rFIX concentrates.
In general, switching product did not reduce the risk of bleeding at personal level, in HA or HB patients. For HA, the risk of bleeding was lower before 1st switch compared to those who never switched. For HB, switching may have reduced the risk of bleeding, but the difference was not significant. It is not clear if bleeding was the driver for HA or HB patients to switch products or it might reflect introduction of new advanced therapies.
Further subgroup analyses may be needed to evaluate the impact of treatment switching pattern in different patient populations, i.e., by patients with and without inhibitors/bypassing agent, in order to assist developing individualized treatment for hemophilia patients.
Disclosures
Gu:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Huang:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Sun:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Zhou:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Shen:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Young:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Chhabra:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Di Russo:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Rupon:Pfizer: Current Employment, Current equity holder in publicly-traded company. Winburn:Pfizer: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal